Travel into the cell
The Cell and Its Components
Cells are the smallest organized structural units able to maintain an individual, albeit limited, life span while carrying out a wide variety of functions. Cells have evolved on earth during the past 3.5 billion years, presumably orginating from suitable early molecular aggregations. Each cell originates from another living cell as postulated by R. Virchow in 1855 (“omnis cellula e cellula”). The living world consists of two basic types of cells: prokaryotic cells, which carry their functional information in a circular genome without a nucleus, and eukaryotic cells, which contain their genome in individual chromosomes in a nucleus and have a well-organized internal structure. Cells communicate with each other by means of a broad repertoire of molecular signals. Great progress has been made since 1839, when cells were first recognized as the “elementary particles of organisms” by M. Schleiden and T. Schwann. Today we understand most of the biological processes of cells at the molecular level.
A. Eukaryotic cells
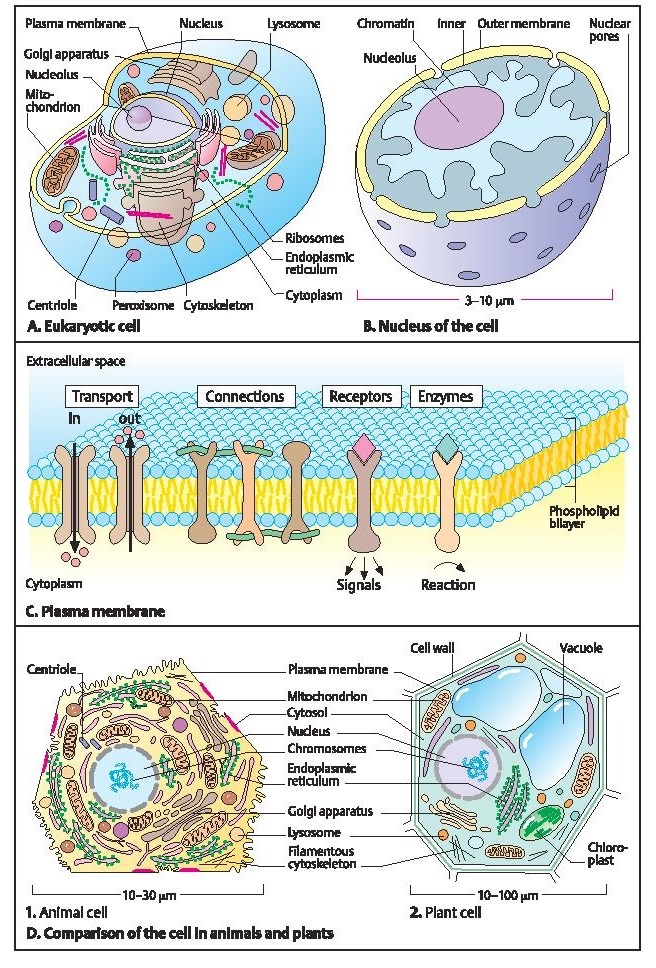
A eukaryotic cell consists of cytoplasm and a nucleus. It is enclosed by a plasma membrane. The cytoplasm contains a complex system of inner membranes that form cellular structures (organelles). The main organelles are the mitochondria (in which important energy–delivering chemical reactions take place), the endoplasmic reticulum (consisting of a series of membranes in which glycoproteins and lipids are formed), the Golgi apparatus (for certain transport functions), and peroxisomes (for the formation or degradation of certain substances). Eukaryotic cells contain lysosomes, in which numerous proteins, nucleic acids, and lipids are broken down. Centrioles, small cylindrical particles made up of microtubules, play an essential role in cell division. Ribosomes are the sites of protein synthesis.
B. Nucleus of the Cell
The eukaryotic cell nucleus contains the genetic information. It is enclosed by an inner and an outer membrane, which contain pores for the transport of substances between the nucleus and the cytoplasm. The nucleus contains a nucleolus and a fibrous matrix with different DNA–protein complexes.
C. Plasma membrane of the cell
The environment of cells, whether blood or other body fluids, is water-based, and the chemical processes inside a cell involve water-soluble molecules. In order to maintain their integrity, cells must prevent water and other molecules from flowing in or out uncontrolled. This is accomplished by a water-resistant membrane composed of bipartite molecules of fatty acids, the plasma membrane. These molecules are phospholipids arranged in a double layer (bilayer) with a fatty interior. The plasma membrane itself contains numerous molecules that traverse the lipid bilayer once or many times to perform special functions. Different types of membrane proteins can be distinguished: (i) transmembrane proteins used as channels for transport of molecules into or out of the cell, (ii) proteins connected with each other to provide stability, (iii) receptor molecules involved in signal transduction, and (iv) molecules with enzyme function to catalyze internal chemical reactions in response to an external signal. (Figure redrawn from Alberts et al., 1998.)
D. Comparison of animal and plant cells
Plant and animal cells have many similar characteristics. One fundamental difference is that plant cells contain chloroplasts for photosynthesis. In addition, plant cells are surrounded by a rigid wall of cellulose and other polymeric molecules and contain vacuoles for water, ions, sugar, nitrogen–containing compounds, or waste products. Vacuoles are permeable to water but not to the other substances enclosed in the vacuoles. (Figures in A, B and D adapted from de Duve, 1984.)
Carbohydrates
Carbohydrates in their various chemical forms and their derivatives are an important group of biomolecules for genetics. They provide the basic framework of DNA and RNA. Their flexibility makes them especially suitable for transferring genetic information from cell to cell.
Along with nucleic acids, lipids, and proteins, carbohydrates are one of the most important classes of biomolecules. Their main functions can be classified into three groups: (i) to deliver and store energy, (ii) to help form DNA and RNA, the information-carrying molecules (see pp. 34 and 38), (iii) to help form cell walls of bacteria and plants. Carbohydrates are often bound to proteins and lipids.
As polysaccharides, carbohydrates are important structural elements of the cell walls of animals, bacteria, and plants. They form cell surface structures (receptors) used in conducting signals from cell to cell. Combined with numerous proteins and lipids, carbohydrates are important components of numerous cell structures. Finally, they function to transfer and store energy in intermediary metabolism.
A. Monosaccharides
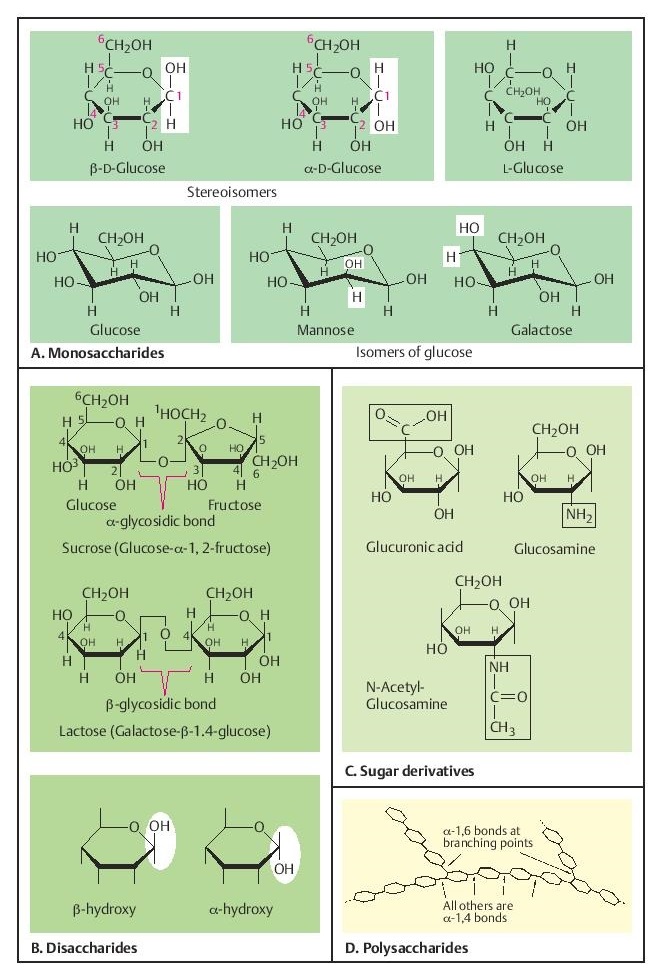
Monosaccharides (simple sugars) are aldehydes (—C=O, —H) or ketones (>C=O) with two or more hydroxy groups (general structural formula (CH2O)n). The aldehyde or ketone group can react with one of the hydroxy groups to form a ring. This is the usual configuration of sugars that have five or six C atoms (pentoses and hexoses). The C atoms are numbered. The D- and the L-forms of sugars are mirror-image isomers of the same molecule.
The naturally occurring forms are the D-(dex-tro) forms. These further include !- and "-forms as stereoisomers. In the cyclic forms the C atoms of sugars are not on a plane, but three-dimensionally take the shape of a chair or a boat. The !-D-glucopyranose configuration (glucose) is the energetically favored, since all the axial positions are occupied by H atoms. The arrangement of the —OH groups can differ, so that stereoisomers such as mannose or galactose are formed.
B. Disaccharides
These are compounds of two monosaccharides. The aldehyde or ketone group of one can bind to Sucrose and lactose are frequently occurring disaccharides.
C. Derivatives of sugars
When certain hydroxy groups are replaced by other groups, sugar derivatives are formed. These occur especially in polysaccharides. In a large group of genetically determined syndromes, complex polysaccharides can not be degraded owing to reduced or absent enzyme function (mucopolysaccharidoses, mucoli-pidoses)
D. Polysaccharides
Short (oligosaccharides) and long chains of sugars and sugar derivatives (polysaccharides) form essential structural elements of the cell. Complex oligosaccharides with bonds to proteins or lipids are part of cell surface structures, e.g., blood group antigens.
Examples of human hereditary disorders in the metabolism of carbohydrates
Diabetes mellitus: a heterogeneous group of disorders characterized by elevated levels of blood glucose, with complex clinical and genetic features
Disorders of fructose metabolism: Three inherited disorders are known: benign fructo-suria, hereditary fructose intolerance with hypoglycemia and vomiting, and hereditary fructose 1,6-bisphosphate deficiency with hypoglycemia, apnea, lactic acidosis, and often lethal outcome in newborn infants.
Glycogen storage diseases: a group of disorders of glycogen metabolism that differ in clinical symptoms and the genes and enzymes involved.
Galactose metabolism: Three different inherited disorders with acute toxicity and long-term effects.
Lipids (Fats)
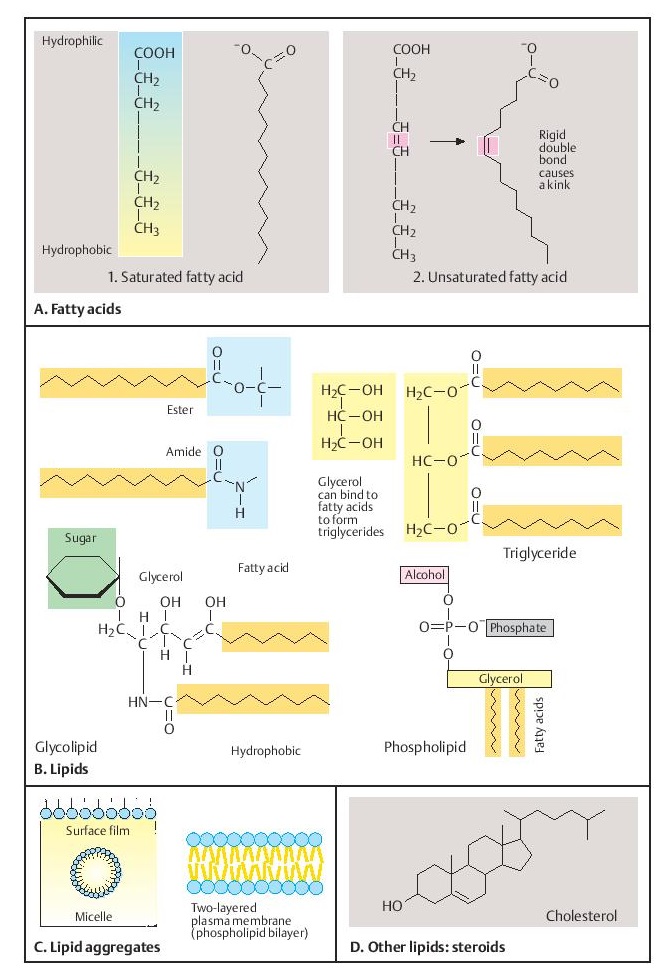
Lipids usually occur as large molecules (macromolecules). They are essential components of membranes and precursors of other important biomolecules, such as steroids for the formation of hormones and other molecules for transmitting intercellular signals. In addition to fatty acids, compounds with carbohydrates (gly-colipids), phosphate groups (phospholipids), and other molecules are especially important. A special characteristic is their pronounced polarity, with a hydrophilic (water-attracting) and a hydrophobic (water-repelling) region. This makes lipids especially suited for forming the outer limits of the cell (cell membrane).
A. Fatty acids
Fatty acids are composed of a hydrocarbon chain with a terminal carboxylic acid group. Thus, they are polar, with a hydrophilic (—COOH) and a hydrophobic end (—CH3), and differ in the length of the chain and its degree of saturation. When one or more double bonds occur in the chain, the fatty acid is referred to as unsaturated. A double bond makes the chain relatively rigid and causes a kink. Fatty acids form the basic framework of many important macromolecules. The free carboxyl group (—COOH) of a fatty acid is ionized (—COO–).
B. Lipids
Fatty acids can combine with other groups of molecules to form other types of lipids. As water-insoluble (hydrophobic) molecules, they are soluble only in organic solvents. The car-boxyl group can enter into an ester or an amide bond. Triglycerides are compounds of fatty acids with glycerol.
Glycolipids (lipids with sugar residues) and phospholipids (lipids with a phosphate group attached to an alcohol derivative) are the structural bases of important macromolecules. Their intracellular degradation requires the presence of numerous enzymes, disorders of which have a genetic basis and lead to numerous genetically determined diseases.
Sphingolipids are an important group of molecules in biological membranes. Here, sphingosine, instead of glycerol, is the fatty acid-binding molecule. Sphingomyelin and gangliosides contain sphingosine. Gangliosides make up 6% of the central nervous system ipids. They are degraded by a series of enzymes. Genetically determined disorders of their catabolism lead to severe diseases, e.g., Tay–Sachs disease due to defective degradation of ganglioside GM2 (deficiency of !-N-acetyl-hexosaminidase).
C. Lipid aggregates
Owing to their bipolar properties, fatty acids can form lipid aggregates in water. The hy-drophilic ends are attracted to their aqueous surroundings; the hydrophobic ends protrude from the surface of the water and form a surface film. If completely under the surface, they may form a micelle, compact and dry within. Phos-pholipids and glycolipids can form two-layered membranes (lipid membrane bilayer). These are the basic structural elements of cell membranes, which prevent molecules in the surrounding aqueous solution from invading the cell.
D. Other lipids: steroids
Steroids are small molecules consisting of four different rings of carbon atoms. Cholesterol is the precursor of five major classes of steroid hormones: prostagens, glucocorticoids, miner-alocorticoids, androgens, and estrogens. Each of these hormone classes is responsible for important biological functions such as maintenance of pregnancy, fat and protein metabolism, maintenance of blood volume and blood pressure, and development of sex characteristics.
Examples of human hereditary disorders in lipoprotein and lipid metabolism
Scriver et al. (2001) list several groups of dis
orders. Important examples are familial hyper-cholesterolemia , familial lipoprotein lipase deficiency, dysbetalipoproteinemia, and disorders of high-density lipoprotein.